
Information on
Non-Muscle
Invasive Bladder
Cancer (NMIBC)
Bladder cancer is the 9th most common cancer type globally. Among newly diagnosed bladder cancer patients, approximately 70% to 80% have Non-Muscle Invasive Bladder Cancer (NMIBC)[3]
For distributors: Discover therapy options
Bladder cancer
in brief
In 2022, more than 610,000 new cases of bladder cancer and 220,000 bladder cancer-related deaths were registered. By 2030, the number of incidents is projected to have climbed to more than 760,000[1].
The risk of contracting bladder cancer increases late in life, and the average age of diagnosis is around 73 years[2].
Men are diagnosed at roughly four times the rate of women, making bladder cancer the 6th most common cancer type among men[1].
Among women, bladder cancer is the 17th most common cancer type. However, studies show that women are more likely to present with muscle invasive bladder cancer (MIBC) upon a first diagnosis[18] due to urinary tract infection, wrong differential diagnosis, and subsequent diagnosis delay[20, 21].
Bladder cancer is the 9th most common cancer type globally1
App. 4 times more men than women are diagnosed1
70% to 80% of newly diagnosed bladder cancer incidents are NMIBC3
From 2022 to 2030, global incidents are projected to increase by more than 24%1
What causes bladder cancer?
As with many types of cancer, one of the leading risk factors is smoking. Those who smoke may be up to four times more likely to contract bladder cancer[2, 22, 23].
People who work with certain chemicals may also be at risk – leather workers, hairdressers, mechanics, and painters, among others[2]. Risk factors also include gene-environment interactions, pollution exposure, and others[24].
However, cancer is a complex disease and it is not always possible to specify its causes. Many people will develop bladder cancer for no known reason[25].
How is bladder cancer diagnosed?
The earlier bladder cancer is diagnosed, the better the outcome of the treatment. Since there is no screening test for bladder cancer available today, most people are diagnosed after experiencing symptoms, such as blood in the urine.
However, as the symptoms of bladder cancer are similar to those of Urinary Tract Infection and other conditions, the diagnosis of bladder cancer can be delayed.
Disease progression and treatment
Approximately 70% to 80% of patients with newly diagnosed bladder cancer will present with NMIBC. Of the patients diagnosed with NMIBC, 50-70% will have a recurring diagnosis despite adequate and timely treatment. Without treatment, 10–20% of patients will progress to Muscle-Invasive Bladder Cancer[2].
Early detection, proper diagnosis, and an adequate treatment regimen, paired with close monitoring are crucial factors to prevent progression from NMIBC to MIBC.
Current guidelines from both the American Urological Association (AUA) and the European Association of Urology (EAU) recommend Transurethral Resection of Bladder Tumor (TURBT) and intravesical BCG as first-line treatment for intermediate- and high-risk NMIBC. TURBT alone is considered insufficient[5,6].
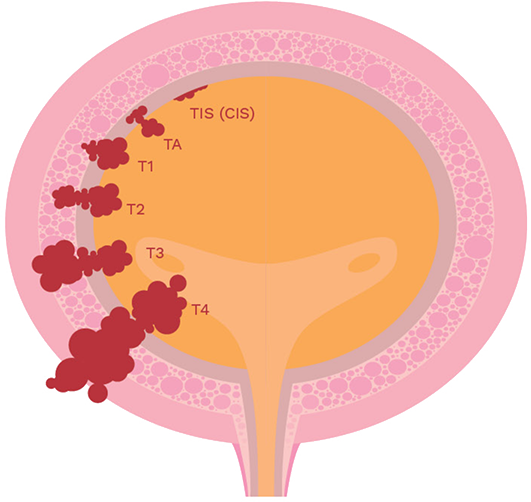
Progression of bladder cancer
stages [7]
Non-Muscle Invasive Bladder Cancer (NMIBC)
Most bladder cancers originate in the transitional epithelial cell lining of the bladder (TIS/CIS, Ta, T1).
Muscle-Invasive Bladder Cancer (MIBC)
If untreated or inadequately treated, NMIBC can progress into muscle-invasive stages (T2, T3), with T4 representing advanced cancer spread to other organs or parts of the body.
High
recurrence and progression
risk if undertreated.
70-80%
of bladder cancer cases are non–muscle-invasive.

Bacillus Calmette-Guérin (BCG)
– a go-to therapy
In the 1970s, studies showed that administration of BCG directly into the bladder reduced recurrence of Non-Muscle Invasive Bladder Cancer. The therapy proved to be effective and safe, and in 1990 it became the first FDA-approved immunotherapy against cancer.
Rather than directly attacking cancer cells, BCG activates the body’s defense system. When introduced into the bladder, it sets off an immune response that mobilizes T cells and other immune cells to eliminate tumor cells from the bladder lining.
According to the European Association of Urology’s (EAU) 2024 guidelines, intravesical immunotherapy with BCG is superior to intravesical chemotherapy in reducing recurrences and in preventing or delaying progression to Muscle Invasive Bladder Cancer (MIBC).
The American Urology Association’s (AUA) guidelines recommend BCG-based therapies as first-line treatment for Intermediate- and High-Risk NMIBC. Both the EAU and the AUA provide guidelines for optimal use of BCG.
BCG therapy procedure
After TURBT surgery and a healing phase, the recommended BCG regimen begins with an induction phase, followed by a maintenance phase[8].
HEALING PHASE: 14-21 days for recovery after surgery.
INDUCTION PHASE: 2-3 weeks after TURBT, BCG is typically administered once a week for 6 weeks to stimulate the immune system to attack remaining cancer cells in the bladder lining. The goal for this repeated regimen is to challenge cancer cells and enhance the immune response against bladder cancer.
MAINTENANCE PHASE: If patients respond well to the initial treatment without serious adverse events, BCG treatment can be resumed during a maintenance phase, typically lasting 1–3 years (subject to regional and national guidelines). This involves a reduced number of treatments to help prevent recurrence, e.g. 3 weekly instillations at 3, 6, and 12 months[8].
Recommended BCG therapy procedure
Turbt
Transurethral Resection of Bladder Tumor
Day 1
Healing Phase
From day 14-21 after TURBT
Day 14
Induction Phase
Typically, 1 weekly BCG instillation
6-week period
Maintenance Phase
Typically, 3 weekly BCG instillations every
3-6 months
1-3 years
For distributors: Login to learn more
Learn more about NMIBC and BCG therapy.
Regulatory/legal disclaimers and references
VesiCulture Abbreviated Prescribing Information
Please refer to the full prescribing information for more details.
C: Live attenuated Mycobacterium bovis BCG, Danish strain 1331 I: Treatment of primary/recurrent flat urothelial cell carcinoma in situ of the bladder; adjuvant treatment after transurethral resection of primary/recurrent superficial urothelial cell carcinoma of the bladder in stage TA/T1, grade 1, 2/3 D: Intravesically Content of 4 vials (120 mg) dissolved in 50 mL saline once/week for 6 weeks CI: Hypersensitivity; active tuberculosis; those receiving anti-tuberculosis drugs; reduced immune response; HIV infection; pregnancy & breast-feeding; medical history including radiation therapy of the bladder SP: Only for instillation in the bladder; not to be used for BCG vaccination; Mantoux test to be performed prior to instillation; defer treatment until healed if macroscopic haematuria, damage to urethra/mucous membranes of the bladder is present; avoid traumatic instillation; infection of implants & transplants has been reported after treatment; monitor for symptoms of systemic BCG infection & toxic symptoms; screen possible HIV-positive patients prior to treatment; abstinence from intercourse/use a condom for one week; may sensitize patients to tuberculin; risk of bladder contraction in patients with low bladder capacity; reactive arthritis/Reiter’s syndrome may increase in patients with tissue type HLA-B27; preparation & administration under antiseptic conditions; do not handle in same room/by personnel preparing cytostatics for IV administration; spillage can cause BCG contamination; personnel may be exposed to BCG through self-inoculation; patients with reduced immune response should avoid contact with patients on BCG treatment AR: Cystitis & inflammatory reactions (granulomas) resulting in pollakiuria & dysuria; malaise; subfebrile; influenza-like symptoms; inflammation of the mucous membranes of the bladder INT: Sensitive to most antibiotics; immunosuppressants; bone marrow suppressants; radiation therapy
References
(1) GLOBOCAN 2022, The International Agency for Research on Cancer (IARC).
(2) The American Cancer Society, Key Statistics for Bladder Cancer, 2024.
(3) Mikolaj Filon, MD, and Bogdana Schmidt, MD, New Treatment Options for Non–Muscle-Invasive Bladder Cancer. Publication: American Society of Clinical Oncology Educational Book Volume 45, Number 2.
(4) Transparency Market Research, Non-muscle Invasive Bladder Cancer Market (Treatment Type: Immunotherapy, Chemotherapy, and targeted Therapy; and Cancer Type: Low Grade Bladder Cancer and High Grade Bladder Cancer) – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2024-2034
(5) American Urological Association, Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline, September 2025.
(6) European Guidelines (EAU): https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Non-muscle-Invasive-Bladder-Cancer-2023_2023-03-10-101110_jued.pdf
(7) World Bladder Cancer Patient Coalition. What is Bladder Cancer factsheet.
(8) Holzbeierlein J, Bixler BR, Buckley DI, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J Urol. 2024;10.1097/JU.0000000000003846.
(9) Miyake, M., et al. Outcomes of subsequent non-muscle-invasive bladder cancer treated with intravesical Bacillus Calmette-Guerin after radical nephroureterectomy for upper urinary tract urothelial carcinoma. BJU Int, 2018. 121: 764. https://www.ncbi.nlm.nih.gov/pubmed/29281857 332.
(10) Rentsch, C.A., et al. Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol, 2014. 66: 677. https://www.ncbi.nlm.nih.gov/pubmed/24674149 333.
(11) Sengiku, A., et al. A prospective comparative study of intravesical bacillus Calmette-Guerin therapy with the Tokyo or Connaught strain for nonmuscle invasive bladder cancer. J Urol, 2013. 190: 50. https://www.ncbi.nlm.nih.gov/pubmed/23376145.
(12) Boehm, B.E., et al. Efficacy of bacillus Calmette-Guerin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. J Urol, 2017. 198: 503. https://www.ncbi.nlm.nih.gov/pubmed/28286068.
(13) Sylvester, R.J., et al. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol, 2002. 168: 1964. https://www.ncbi.nlm.nih.gov/pubmed/12394686.
(14) Unda-Urzaiz, M., et al. Safety and efficacy of various strains of bacille Calmette-Guerin in the treatment of bladder tumours in standard clinical practice. Actas Urol Esp (Engl Ed), 2018. 42: 238. https://www.ncbi.nlm.nih.gov/pubmed/29295749.
(15) Steinberg, R.L., et al. Bacillus Calmette-Guerin strain may not effect recurrence-free survival when used intravesically with interferon-alpha2b for non-muscle-invasive bladder cancer. Urol Oncol, 2017. 35: 201. https://www.ncbi.nlm.nih.gov/pubmed/28041998.
(16) https://uroweb.org/guidelines/non-muscle-invasive-bladder cancer.
(17) Summary of Product characteristics (SmPC) for Vesiculture.
(18) Scheller, T.; Hofmann, R.; Hegele, A. Sex-related differences in urothelial cell carcinoma of the bladder in Germany. Cancer Manag. Res. 2019, 11, 309–316.
(19) Poli, C.; Trétarre, B.; Trouche-Sabatier, S.; Foucan, A.S.; Gras-Aygon, C.; Abdo, N.; Poinas, G.; Azria, D.; Rébillard, X.; Iborra, F. Sex differences in muscle-invasive bladder tumors: A study of a French regional population. Fr. J. Urol. 2024, 5, 102723.
(20) A Soave, R Dahlem, J Hansen, L Weisbach, S Minner, O Engel, L A Kluth, F K Chun, S F Shariat, M Fisch, M Rink.. Gender-specific outcomes of bladder cancer patients: a stage-specific analysis in a contemporary, homogenous radical cystectomy cohort. Eur J Surg Oncol. 2015 Mar;41(3):368-77.
(21) Géraldine Pignot, Philippe Barthélémy, Delphine Borchiellini. Sex Disparities in Bladder Cancer Diagnosis and Treatment. Cancers (Basel). 2024 Dec 7;16(23):4100.
(22) Neal D Freedman, Debra T Silverman, Albert R Hollenbeck, Arthur Schatzkin, Christian C Abnet. Association between smoking and risk of bladder cancer among men and women. JAMA. 2011 Aug 17;306(7):737–745.
(23) Lei Xiang, Qi-Qi Xie, Si-Si Xu, Wen-Jie Ruan, Dong-Hui Xu, Yao-Yao Gan, Jia Zuo, Wen-Jun Xu, Zhi-Peng Li. Association between tobacco exposure and bladder cancer recurrence: A systematic review and meta-analysisWorld J Methodol. 2024 Jun 20;14(2):91889.
(24) Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Jubbler, Eur UroL 2023 Aug;84(2):176-190. doi: 10.1016/j.eururo.2023.03.029. Epub 2023 May 16.
(25) RiskFactorsAssociated with UrothelialBladderCancer. Alouini S.Int J Environ Res Public Health. 2024 Jul 22;21(7):954.
(26) AJ Vaccine’s Pharmacovigilance data base and Periodic Safety Update Report (PSUR) for VesiCulture.
(27) Abou Chakra M, Luo Y, Duquesne I, A O’Donnell M. Update on the Mechanism of Action of Intravesical BCG Therapy to Treat Non-Muscle-Invasive Bladder Cancer. Front Biosci (Landmark Ed). 2024 Aug 21;29(8):295. doi: 10.31083/j.fbl2908295.
(28) Lamm D.L. Efficacy and safety of Bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31.
(29) Brausi M., Oddens J., Sylvester R., Bono A., van de Beek C., van Andel G. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014.
(30) van der Meijden A.P.M., Sylvester R.J., Oosterlinck W., Hoeltl W., Bono A.V. Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a european organisation for research and treatment of cancer genito-urinary group phase III trial. Eur Urol. 2003;44(4):429–434
(31) M D Shelley, J B Court, H Kynaston, T J Wilt, B Coles, M Mason. Intravesical bacillus Calmette-Guerin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2003:(3)
(32) Dickon Hayne, Martin Stockler, Steve P McCombie, Venu Chalasani, Anne Long, Andrew Martin, Shomik Sengupta, Ian D Davis. BCG+MMC trial: adding mitomycin C to BCG as adjuvant intravesical therapy for high-risk, non-muscle-invasive bladder cancer: a randomised phase III trial (ANZUP 1301). BMC Cancer. 2015 May 27:15:432.
(33) CANCER TOMORROW, World Health Organization, International Agency for Research on Cancer, 2025, https://gco.iarc.who.int/tomorrow/en